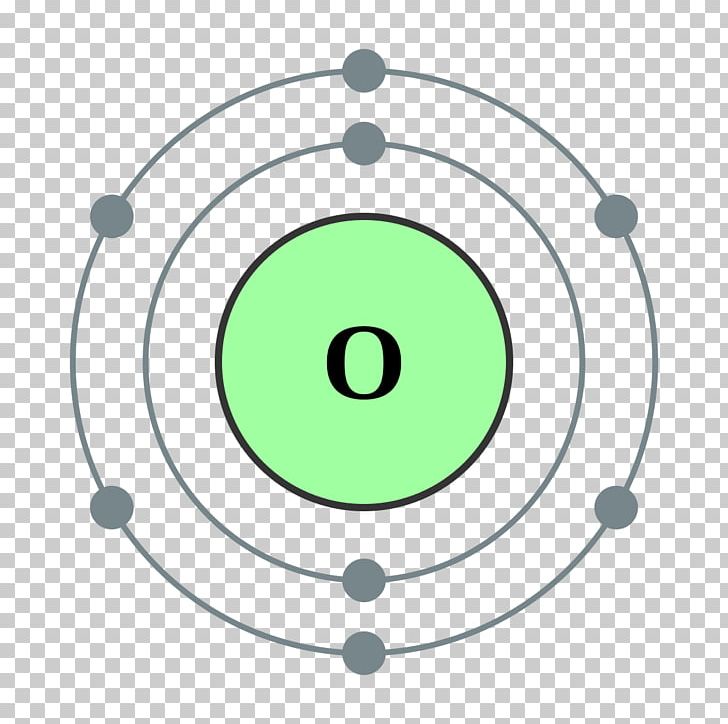
Bohr Model Chemical Element Oxygen Atomic Theory PNG, Clipart, Angle, Area, Atom, Atomic Number
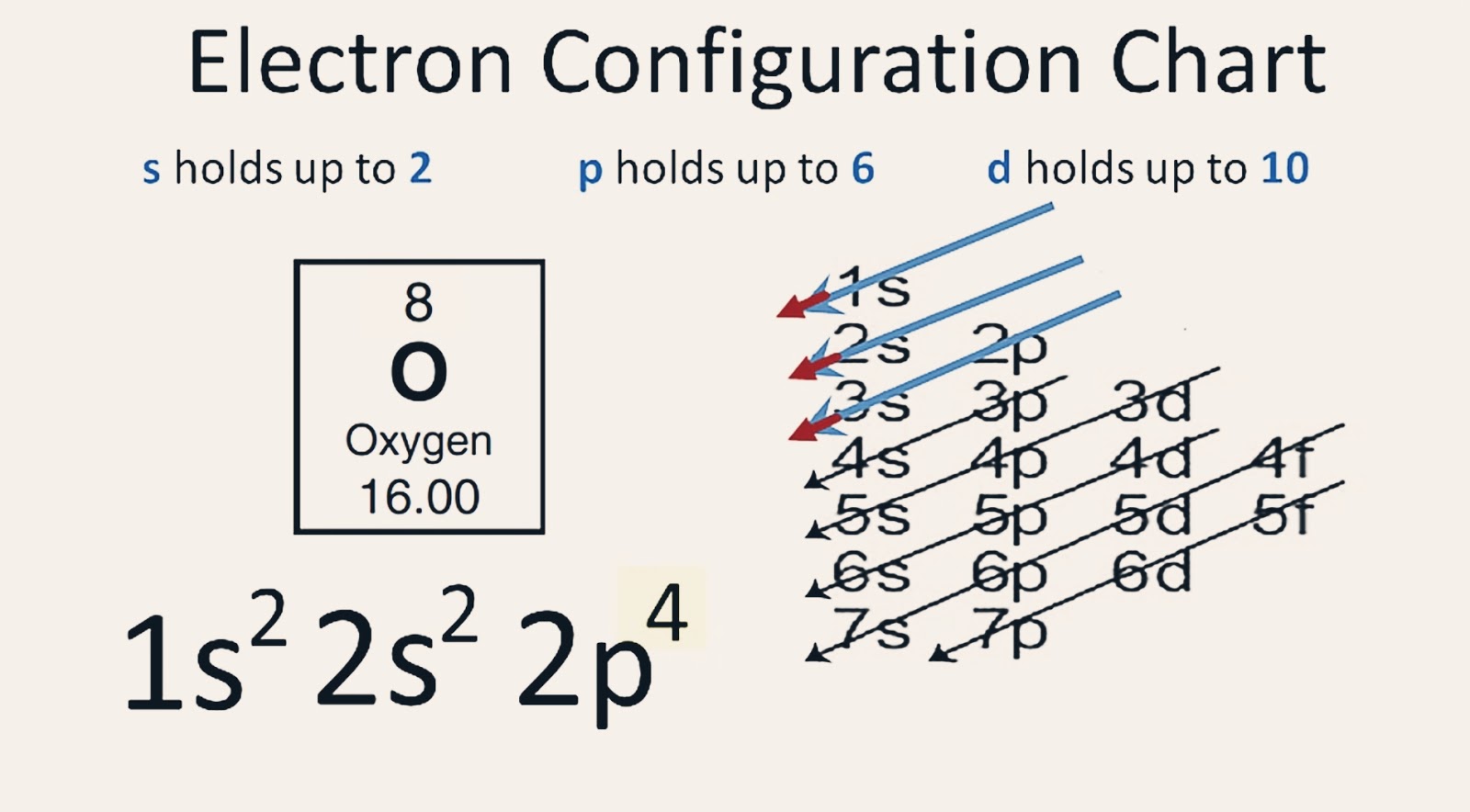
Ground State Electron Configuration of Oxygen. The way electrons are arranged in oxygen is shown by the numbers 1s^2, 2s^2, 2p^4. This tells us how many electrons are in each part. Let's break it down and explain it more simply. Oxygen has eight electrons. The first energy level can hold two electrons, and oxygen has two at this level.
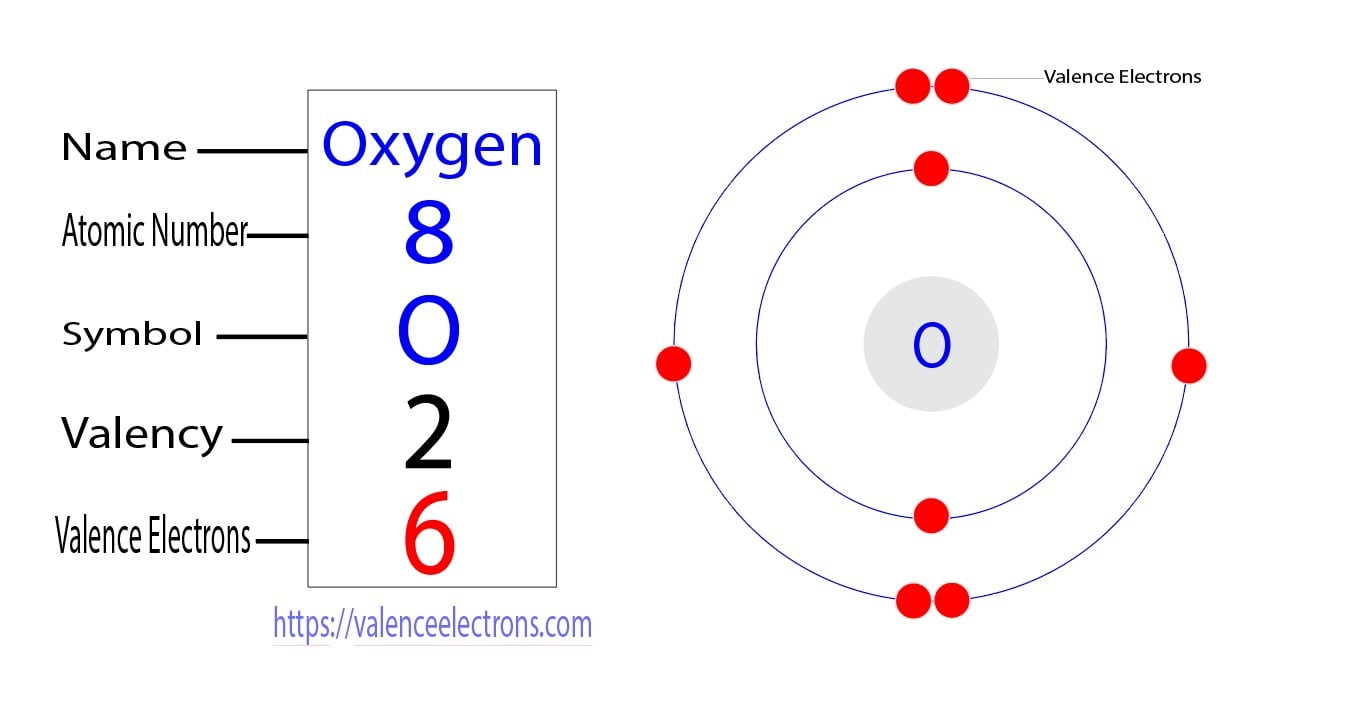
Electron Configuration for Oxygen (O, and O2 ion)
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period table or an electron configuration chart. How to Write the Electron Configuration for Oxygen Oxygen is the eighth element with a total of 8 electrons.

Electronic configuration of the oxygen atom Download Scientific Diagram
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence.

The electron configuration of oxygen is 1s2,2s2 2p4. Science chemistry, Electron configuration
Let's find the electron configuration of Oxygen! A single oxygen atom has 8 protons and 8 electrons, but how do we know where Oxygen puts its electrons, in w.
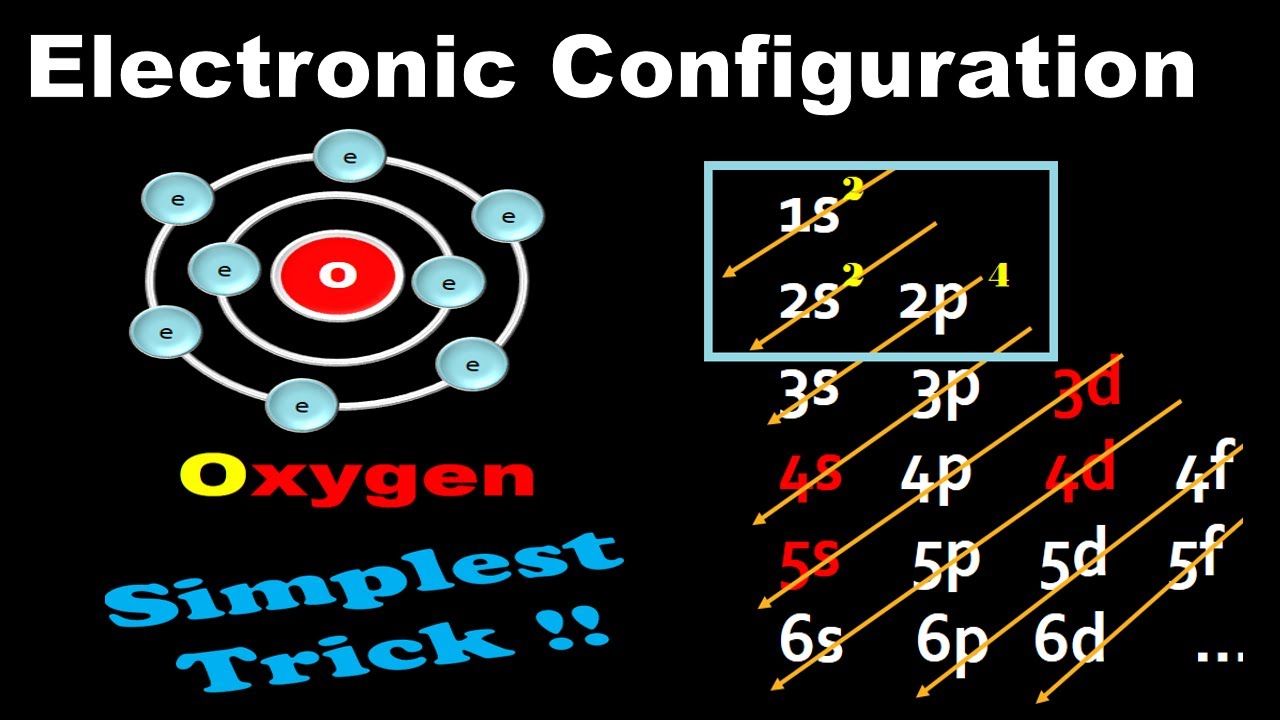
Electronic Configuration For Oxygen spdf Trick Chemistry Atomic Number 8 YouTube
The electronic configurations of atoms close atom The smallest part of an element that can exist. help explain the properties of elements and the structure of the periodic table.
How to Write Ground State Electron Configuration in Chemistry
We expect the two electrons that occupy these two degenerate orbitals to be unpaired, and this molecular electronic configuration for O 2 is in accord with the fact that the oxygen molecule has two unpaired electrons ( Figure \(\PageIndex{10}\)). The presence of two unpaired electrons has proved to be difficult to explain using Lewis structures, but the molecular orbital theory explains it.
What Is the Oxygen Electron Configuration(O)?
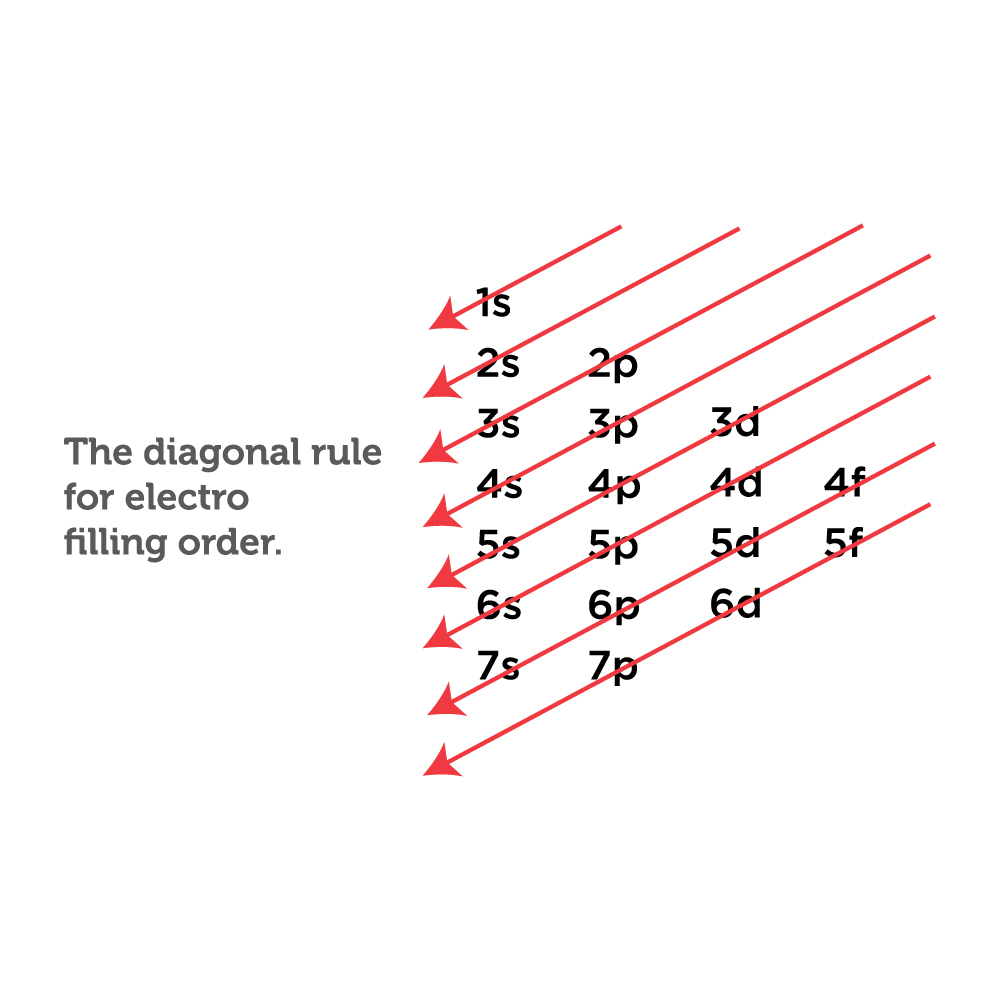
The easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. It looks something like this.
What is the Electron Configuration of Oxygen Archives Dynamic Periodic Table of Elements and
Oxygen Electron Configuration Wayne Breslyn 724K subscribers Join Subscribe Subscribed 683 Share 130K views 10 years ago A step-by-step description of how to write the electron configuration.

Diagram representation of the element oxygen Vector Image
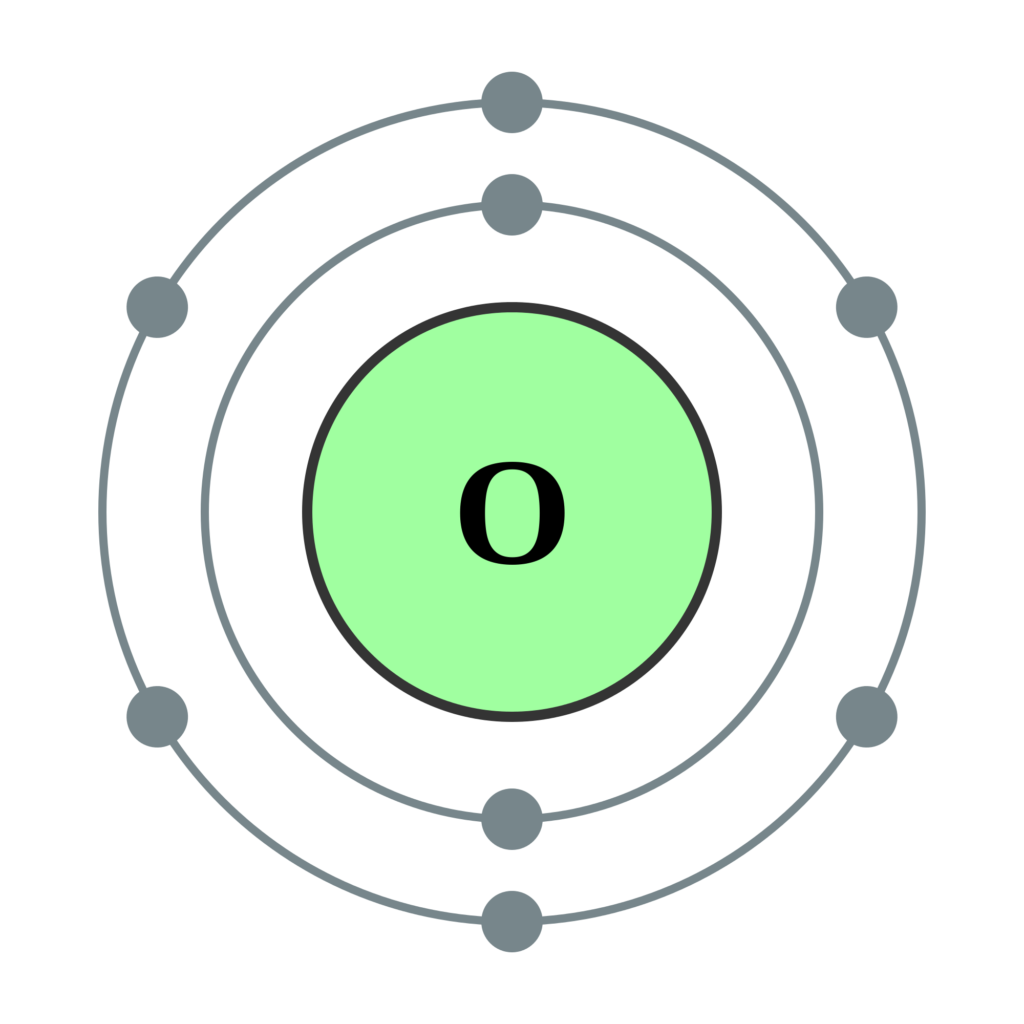
An electronic configuration is the way in which electrons are arranged in an atom . Electrons in shells Different shells can hold different maximum numbers of electrons. Electrons.

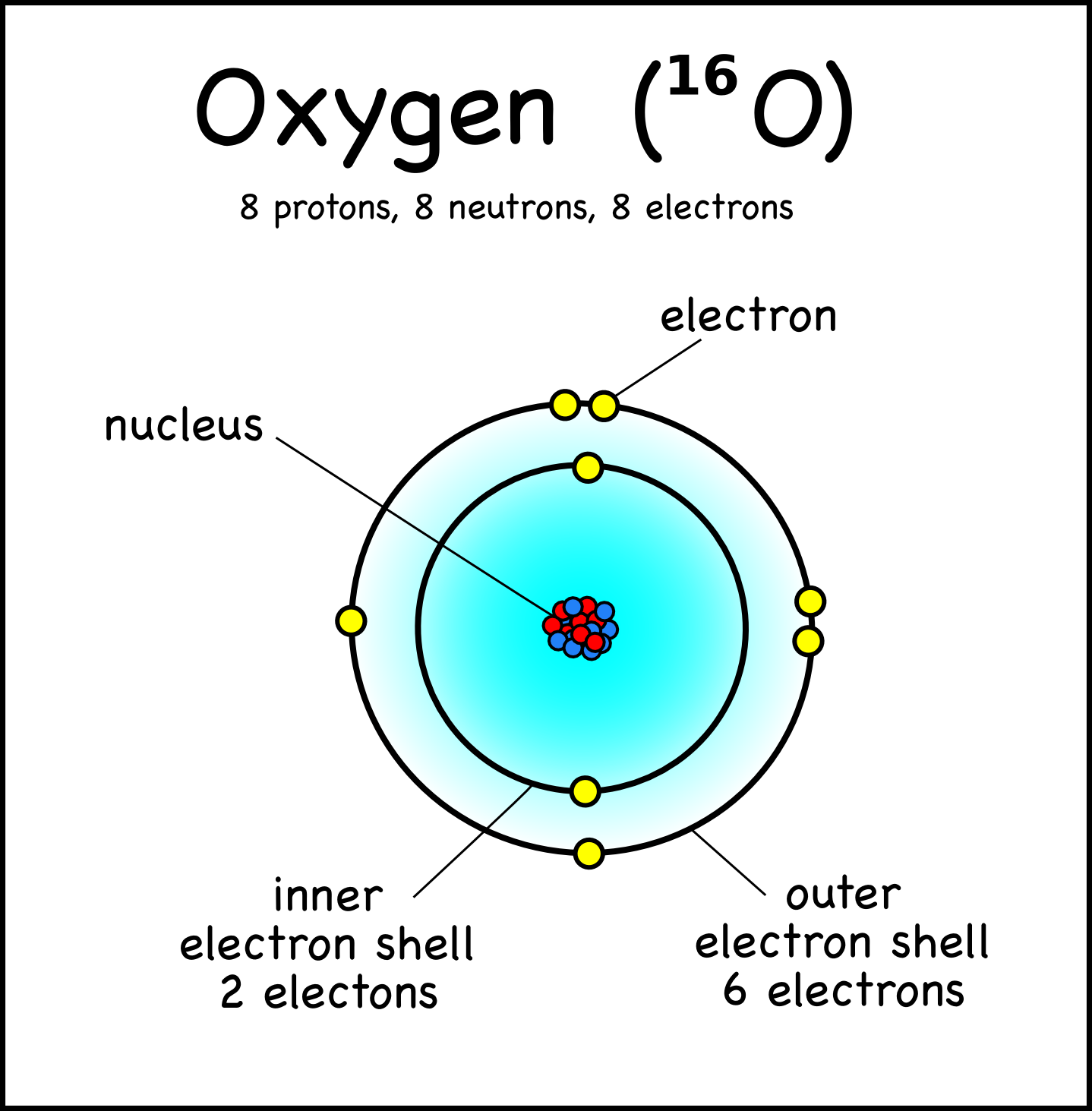
Oxygen Atom Science Notes and Projects
Solution Oxygen: Oxygen is an element having an atomic number 8 and an atomic symbol O. It belongs to Group- 16 and second period. It is a highly reactive non-metal. Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration.
Bohr Model Drawing Of Oxygen at GetDrawings Free download
To find the electron configuration of oxygen: Look at the periodic table and find an atomic number of oxygen, which is 8. Fill these 8 electrons in the following order: 1s, 2s, and then 2p. Write the complete electron configuration of oxygen: 1s²2s²2p⁴. Identify the noble gas before oxygen, helium, and write using shorthand notation: [He.

Electron Configuration for Oxygen (O, O2 ion)
The electron configuration of oxygen is 1s 2 2s 2 2p 4. In O 2, therefore, we need to accommodate twelve valence electrons (six from each oxygen atom) in molecular orbitals. As you can see from the diagram, this places two electrons in antibonding orbitals. Each of these electrons occupies a separate π* orbital because this leads to less.

FileElectron shell 008 Oxygen.svg Wikimedia Commons Atom diagram, Electron configuration
Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain.
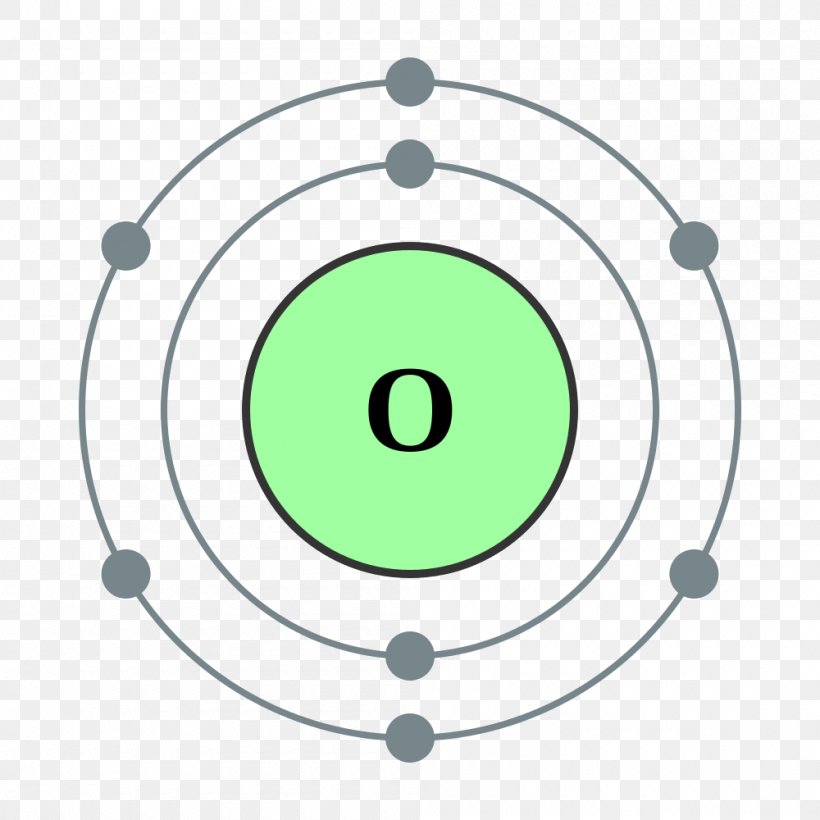
Bohr Model Oxygen Chemical Element Atomic Number, PNG, 1000x1000px, Bohr Model, Area, Atom
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.

Electron configuration of oxygen ion Lousiana
The arrangement of electrons in oxygen in specific rules in different orbits and orbitals is called the electron configuration of oxygen. The electron configuration of oxygen is [ He] 2s 2 2p 4, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)

Electron configuration of oxygen ion Lousiana
Oxygen - Element information, properties and uses | Periodic Table Pressure and temperature data - advanced Young's modulus (GPa) Shear modulus (GPa) Bulk modulus (GPa) , the magazine of the Royal Society of Chemistry. O, just about the most perfect solvent you can imagine for biochemistry.